Standard Volume Calculation of Biogas
Measuring the volume of biogas produced and consumed is an important parameter to measure the efficiency of the biogas plant. In this blog post, we will discuss the standard volume and how it helps to have comparable values for different operating conditions.
Measuring the volume is a practical way to obtain information on this parameter.
However, the volume of gases is dependent on temperature and pressure. The influence of these factors is much greater than with liquids. Humidity, i.e. the proportion of water vapour, also has an influence on the volume.
Simply put, the volume of the gas increases with increasing temperature. If the pressure increases, the volume decreases. However, the mass of the gas remains constant. However, the mass of the gas is actually the decisive variable for describing both biogas production and biogas consumption.
In order for the measured volume flows to be comparable, a definition is therefore required that considers the different operating conditions (temperature, pressure).
The gas laws describe the influences of temperature and pressure for ideal gases. For the low pressures and temperatures that are usually present in biogas, it is possible to work with these relationships.
The Law of Gay-Lussac
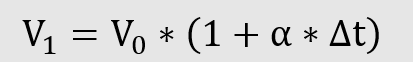

Gay-Lussac’s temperature law describes the uniform expansion of ideal gases during temperature changes, provided the pressure remains constant. The coefficient of expansion α indicates the change in volume, which is 1/273 per degree.
Law of Charles
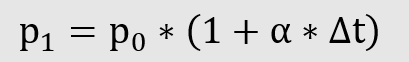
Charles’ law describes the change in pressure at constant volume changed proportionally to the temperature.
Laws of Boyle and Mariotte
The laws of Boyle and Mariotte describe the dependence on temperature, pressure and volume,
1. The product of pressure and volume is constant for ideal gases.
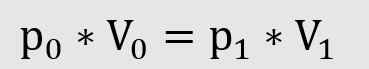

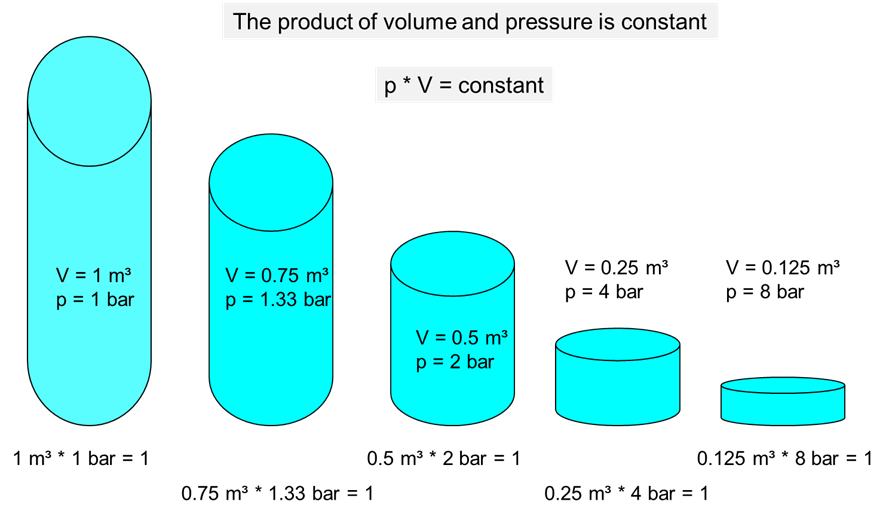
2. The product of temperature and volume is constant for ideal gases
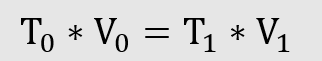

3. The product of pressure and temperature is constant for ideal gases
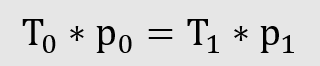

From this can be derived the
equation of state for ideal gases
can be derived.
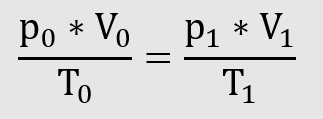

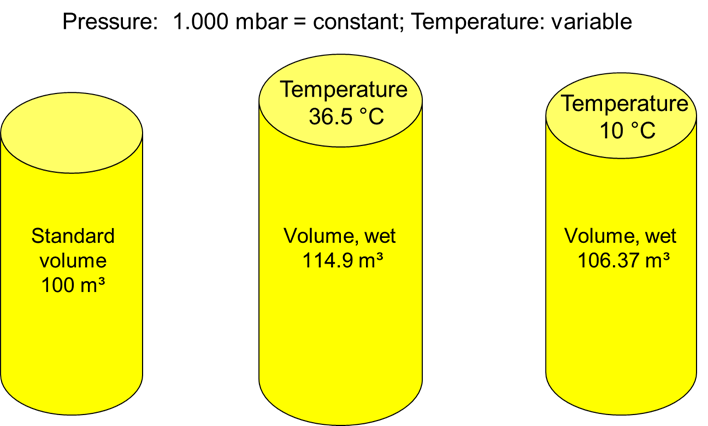
Norm Volume
The standard conditions for the volume mentioned at the beginning are defined as “norm volume” in the German and European areas.
Standard reference conditions – norm temperature and norm pressure
Definition German Standard DIN 1343
The standard condition is the reference condition defined by the norm temperature
Tn = 273,15 K or tn = 0 °C
and the norm pressure
pn = 101 325 Pa = 1,01325 bar
is defined.
The volume of a substance in the standard state, i.e. at the normal temperature and the normal pressure, is called the
norm volume Vn
is called.
The unit is m³ without further additions.
Standard Volume
The standard volume is defined in ISO 2533 and differs from the norm volume by a different reference temperature. The reference temperature is 15 °C.
The standard volume is the reference volume defined by the standard temperature
TSTP = 288.15 K or tSTP = 15 °C
and the standard pressure
pSTP = 101 325 Pa = 1.01325 bar
is defined.
Conversion of operating volume to standard volume
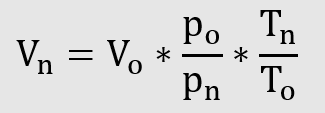

Conversion of standard volume to operating volume
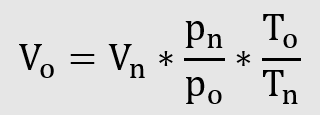
Example

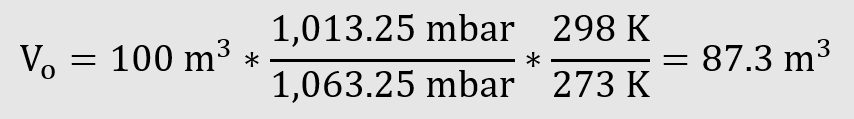

Norm volume / standard volume
Vn 100 m³ (DIN 1343)
Tn 273 K (0 °C)
TSTP 288 K (15 °C)

The standard volume according to ISO 2533 is 5.5 % larger than the norm volume according to DIN 1343. The effect is based exclusively on the slightly higher temperature.
These relationships only apply to dry gases. However, the gas humidity can also be taken into account.
Conversion of operating volume to standard volume taking into consideration the
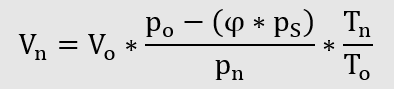

Conversion of standard volume into operating volume taking into consideration the Water vapour partial pressure
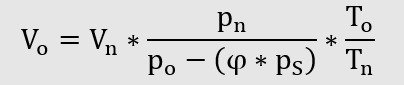
Gas composition and partial pressure
The gas composition is usually given on the dry gas.
- However, the gas is usually saturated with water vapour.
- The proportion of water vapour depends on the temperature.
Gas composition, dry: 50 vol. % methane
50 vol. % carbon dioxide

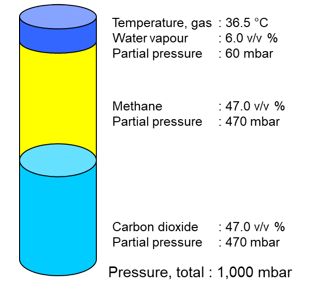
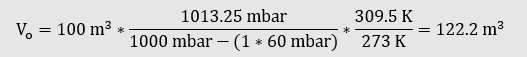
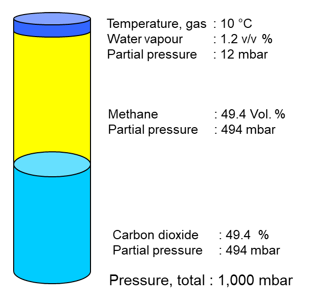
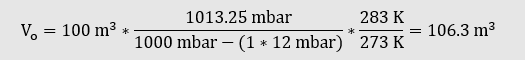
In relation to the dry composition, the temperature of the proportion of water vapour has a significant influence on the real volume of the gas.
Example
A digester with a fixed roof has a gas space with a geometric volume of 100 m³. The pressure in the gas space is 30 mbar, the temperature 35 °C, the gas is saturated with water vapour. The air pressure is 1,020 mbar.
- What is the standard volume?
- How much gas can be used if the pressure in the digester is reduced to 15 mbar?
The equation of state for ideal gases is used first:
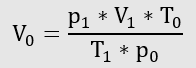

The next step in the calculation is done with the law of Boyle and Mariotte
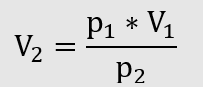

When the pressure is reduced by 15 mbar, 10.2 m³ of gas can be used under operating conditions.
Example
The following example shows the change in gas volume in the different treatment stages in a biogas plant.
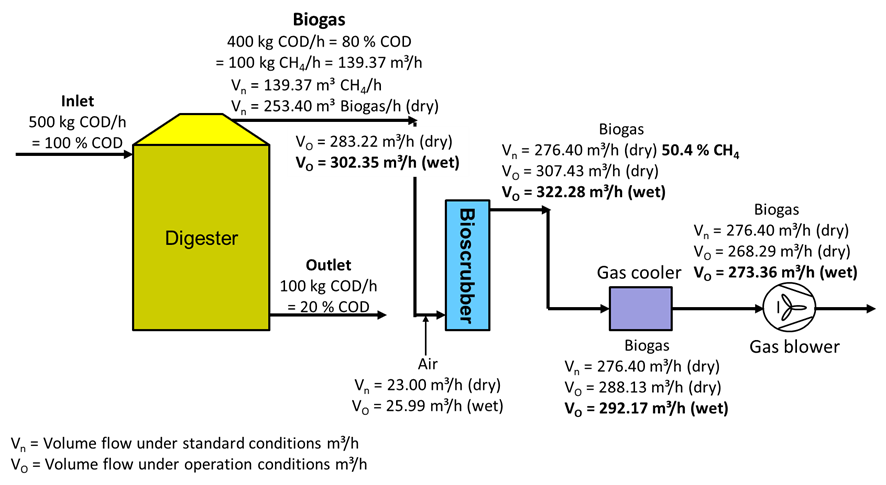
| Gas production | |||
| Composition biogas | : | 55 % CH4, 45 % CO2 | |
| Mass flow methane | : | 100 | kg/h |
| Standard volume flow methane | : | 139.37 | m³/h |
| Standard volume flow biogas | : | 253.40 | m³/h |
| Operating conditions | : | Outlet digester | |
| Temperature biogas | : | 38 | °C |
| Overpressure biogas | : | 20 | mbar |
| Air pressure | : | 1,025 | mbar |
| Relative water vapour saturation | : | 100 | % |
| Operating conditions | : | Outlet bioscrubber | |
| Temperature biogas | : | 32 | °C |
| Overpressure biogas | : | 5 | mbar |
| Air pressure | : | 1,025 | mbar |
| Relative water vapour saturation | : | 100 | % |
| Operating conditions | : | Air bioscrubber | |
| Temperature biogas | : | 30 | °C |
| Overpressure air | : | 25 | mbar |
| Air pressure | : | 1,025 | mbar |
| Relative water vapour saturation | : | 100 | % |
| Operating conditions | : | Outlet gas cooler | |
| Temperature biogas | : | 12 | °C |
| Overpressure biogas | : | 2 | mbar |
| Air pressure | : | 1,025 | mbar |
| Relative water vapour saturation | : | 100 | % |

0 Comments